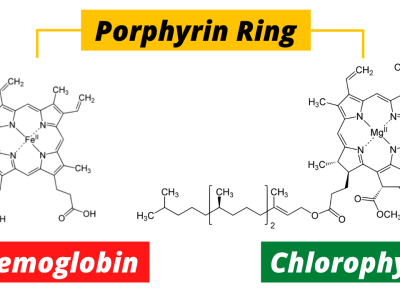
The Green and Red of Life: Chlorophyll vs. Hemoglobin
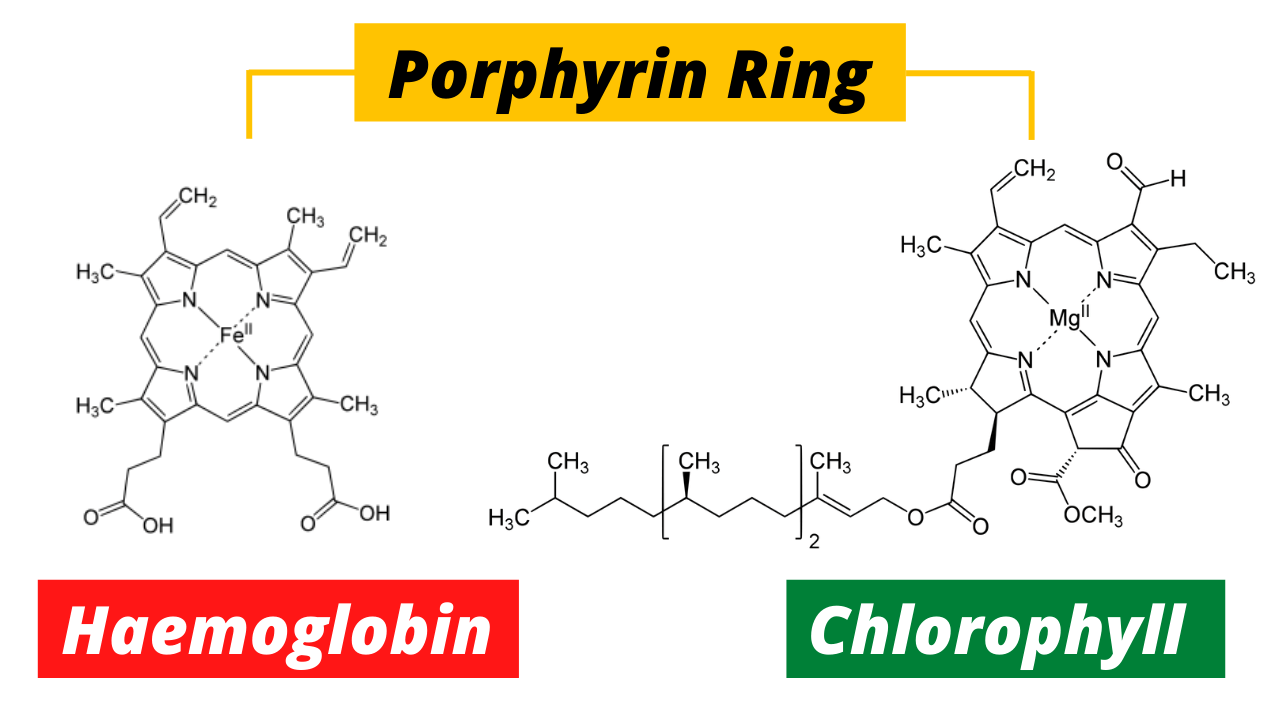
In the grand tapestry of life, two molecules stand as pillars of existence, silently working to power the living world: chlorophyll and hemoglobin. Though they exist in different realms—plants and animals—these two compounds share remarkable similarities. Yet, it’s the profound difference between them that keeps our world in balance. One turns sunlight into energy; the other fuels the breath of life. Their differences, however, come down to a single atom—magnesium versus iron.
Let’s dive into the fascinating connection between these two molecules and uncover how a small change at the atomic level determines the color of life itself.
The Green Engine of Plants: Chlorophyll
Imagine a world without green. It’s almost impossible because nearly every glimpse of nature, from the towering forests to the simplest blade of grass, thrives on one molecule: chlorophyll.
Chlorophyll is the key to the process of photosynthesis, a biochemical magic trick that transforms sunlight into food. Found in the chloroplasts of plant cells, it works like a solar panel, absorbing sunlight, specifically blue and red wavelengths, and reflecting the green light that gives plants their distinctive hue.
At the heart of this molecule lies magnesium (Mg), a metal responsible for this lush green. The structure of chlorophyll is a porphyrin ring—a complex array of carbon and nitrogen atoms—with magnesium sitting snugly in the center. This magnesium atom plays a crucial role in capturing light energy, which is then converted into chemical energy in the form of glucose, fueling the entire plant world and, indirectly, the animal kingdom as well.
Without chlorophyll, life on Earth as we know it would collapse. It is not just the lungs of the planet; it is its very engine.
The Red River of Life: Hemoglobin
On the other side of the biological spectrum is hemoglobin, the molecule that courses through the veins of almost every living creature. While chlorophyll helps plants harness energy, hemoglobin ensures that animals, including humans, can use that energy efficiently.
Hemoglobin’s job is simple, yet crucial—it transports oxygen from the lungs to every cell in the body and brings carbon dioxide back to be expelled. It accomplishes this task through a unique structure. Much like chlorophyll, hemoglobin contains a porphyrin ring, but the central atom in this case is iron (Fe), not magnesium. This difference in metals makes all the difference: while magnesium makes chlorophyll green, iron gives hemoglobin its characteristic red color.
The role of iron in hemoglobin is much more than aesthetic. It is the iron atom that binds oxygen in the lungs and releases it in the tissues. With every heartbeat, hemoglobin delivers the oxygen we need to live, supporting every process from breathing to thinking. Its efficiency and precision are a marvel of biological engineering.
Two Molecules, Two Worlds
Chlorophyll and hemoglobin share a core structure, yet a subtle difference in their makeup defines their function. This single variation—the replacement of magnesium with iron—transforms their roles entirely. While chlorophyll captures energy from the sun, hemoglobin distributes oxygen to cells that need it.
But what’s truly remarkable is that both molecules are critical to life on Earth. Chlorophyll sustains the food chains of the world, feeding not just plants but herbivores, carnivores, and omnivores alike. Hemoglobin, on the other hand, ensures that oxygen, the spark of life, reaches every corner of the body, powering movement, thought, and growth.
Without chlorophyll, there would be no plants. Without hemoglobin, there would be no animals. Together, they form a delicate symbiotic relationship between the green and red of life.
The Power of a Single Atom
It’s easy to overlook just how much hinges on tiny details in nature. A single atom swap, magnesium for iron, turns green leaves into red blood, transforms sunlight into breath. It’s these small differences that shape life itself. And yet, despite their distinct roles, these two molecules underscore one of nature’s deepest truths: life, in all its forms, is connected. Whether we’re admiring the vibrant green of a forest or feeling the pulse in our wrist, the same fundamental chemistry is at work.
Next time you take a breath, or walk through a park, remember the invisible engines of life that keep the planet spinning. The green chlorophyll in the plants around you and the red hemoglobin in your veins are, in essence, two sides of the same coin—one harnessing the sun’s energy, the other making sure we use it. Together, they sustain a world in perfect balance.